However, MMP-mediated controlled proteolysis of the ECM, also releases protein fragments such as endostatin, canstatin, tumstatin, and endorepellin that are biologically active and PF-04217903 potent angiogenesis inhibitors. For a number of years, the tumor-inhibitory and anti-angiogenic properties of TIMPs were believed to be entirely due to their MMP inhibitory properties. As a consequence, there has been a considerable investment of resources to develop safe and effective therapeutic modalities that target MMPs. Several generations of synthetic MMP inhibitors were tested in phase III clinical trials in humans but were found to be surprisingly ineffective relative to the results obtained in preclinical trials. More recently, TIMPs have been shown to be multifunctional proteins with a number of biological activities that were independent of their MMP inhibitory properties. Inhibition of angiogenesis by TIMP-2 and TIMP-3 has been demonstrated to be independent of their ability to inhibit MMPs. Previously described studies of the structure-functional analyses of TIMP-2 revealed that the anti-angiogenic activity of TIMP-2 was present in the C-terminal end specifically in a smaller, 2.9 kDa domain in this region. Based 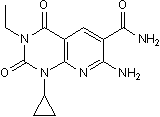 on these studies we designed experiments to determine the region of TIMP-3 that was responsible for angiogenesis inhibition. In the present study we identified the C-terminal region of the protein to be responsible for this effect, using the property of TIMP-3 to block binding of VEGF to VEGFR-2 that we had previously reported. Short peptides were designed based on the functional domains of TIMP1. We mapped the anti-angiogenic activity of TIMP-3 to peptides in the putative Loop 6 and Tail regions of the protein. Peptides based on the Loop 5 and N-terminal domains had no angio-inhibitory activity in vitro or in vivo. Concomitantly, Loop6 and Tail peptides were effective in inhibiting laserinduced CNV in vivo. Sorsby fundus dystrophy, an autosomal dominant, fully penetrant, degenerative disease of the macula is manifested by symptoms of night blindness or sudden loss of acuity, usually in the third to fourth decades of life due to submacular neovascularization. SFD is caused by specific mutations in the tissue inhibitor of metalloproteinases-3 gene, most of which introduce an unpaired cysteine at the C-terminus of the protein. We have recently reported that S156C mutation in TIMP-3 induces increased angiogenesis and that mice lacking TIMP-3 show increased laser-induced CNV. Since the anti-angiogenic activity of TIMP-3 lies in the C-terminus region of the protein and most of the new cysteines in SFD mutations lie in the same region we hypothesized that Loop 6 and Tail peptides of TIMP-3 might be critical determinants of this inhibitory activity. Whether the free cysteine in the tail peptide sequence is critical for the angiogenesis inhibition will be an interesting question to address in Tubacin future studies. Sequence comparison and alignment between a short peptide sequence of pigment epithelial growth factor that shows anti-angiogenic activity and TIMP-3 shows a short consensus sequence of SNFGYXXY between the two proteins. Both PEDF and TIMP-3 are anti-angiogenic proteins whose peptides have been shown to play a critical role in inhibiting ocular angiogenesis especially choroidal neovascularization. Finding consensus sequences in these peptides might provide clues regarding the mechanisms of inhibition of neovascularization.
on these studies we designed experiments to determine the region of TIMP-3 that was responsible for angiogenesis inhibition. In the present study we identified the C-terminal region of the protein to be responsible for this effect, using the property of TIMP-3 to block binding of VEGF to VEGFR-2 that we had previously reported. Short peptides were designed based on the functional domains of TIMP1. We mapped the anti-angiogenic activity of TIMP-3 to peptides in the putative Loop 6 and Tail regions of the protein. Peptides based on the Loop 5 and N-terminal domains had no angio-inhibitory activity in vitro or in vivo. Concomitantly, Loop6 and Tail peptides were effective in inhibiting laserinduced CNV in vivo. Sorsby fundus dystrophy, an autosomal dominant, fully penetrant, degenerative disease of the macula is manifested by symptoms of night blindness or sudden loss of acuity, usually in the third to fourth decades of life due to submacular neovascularization. SFD is caused by specific mutations in the tissue inhibitor of metalloproteinases-3 gene, most of which introduce an unpaired cysteine at the C-terminus of the protein. We have recently reported that S156C mutation in TIMP-3 induces increased angiogenesis and that mice lacking TIMP-3 show increased laser-induced CNV. Since the anti-angiogenic activity of TIMP-3 lies in the C-terminus region of the protein and most of the new cysteines in SFD mutations lie in the same region we hypothesized that Loop 6 and Tail peptides of TIMP-3 might be critical determinants of this inhibitory activity. Whether the free cysteine in the tail peptide sequence is critical for the angiogenesis inhibition will be an interesting question to address in Tubacin future studies. Sequence comparison and alignment between a short peptide sequence of pigment epithelial growth factor that shows anti-angiogenic activity and TIMP-3 shows a short consensus sequence of SNFGYXXY between the two proteins. Both PEDF and TIMP-3 are anti-angiogenic proteins whose peptides have been shown to play a critical role in inhibiting ocular angiogenesis especially choroidal neovascularization. Finding consensus sequences in these peptides might provide clues regarding the mechanisms of inhibition of neovascularization.