A previous study reported that the odds for a drug to stay unchanged during a six month period were greater if prescribed via the MDD system than via ordinary prescriptions. This measure may be seen as a surrogate variable for drug treatment reconsideration. Our results indicate a causal relationship between MDD and fewer changes in drug treatment; after adjustments for relevant covariates, the predicted change in the number of drugs at the index date, compared with the preceding measure date, was significantly smaller when data after the transition was used for the estimations. Underlying mechanisms for our findings can only be speculated upon. Some previous studies indicate that an MDD system may reduce medication errors and provide a better overview of a patient��s medication list. Thus, it cannot be excluded that the medication lists in the present study may be appropriate at the individual level, although extended and more often potentially harmful according to indicators. Other studies, on the other hand, indicate the opposite, namely that medication errors are as common or even more common for patients with MDD. Indeed, MDD was the main risk factors for medication errors at transitions in healthcare.In the past, the original toxin isolated from Shigella dysenteriae has been referred to as Stx, the highly related form isolated from E. coli has been referred to Stx1, and Stx2 has been used to refer to the highly potent form isolated from E. coli. However, numerous polymorphic forms of Stx2 have now been described which can share over 90% amino acid identity, but vary in potency by several orders of magnitude. As more variants have been sequenced, the historic 9-methoxycamptothecine nomenclature has become extremely ambiguous. To avoid confusion, we will refer to the family members as Stx1 and Stx2, and variants used in this study 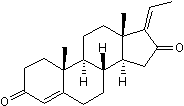 as Stx1-S and Stx2a. STEC can express one, or both forms of toxin. The reduced potency of Stx1 compared to Stx2a is well documented in mice and primates. Furthermore, Stx2a is more commonly associated with lifethreatening human disease; the majority of cases of HUS are associated with strains that produce Stx2a. Similarly, a recent report by Mukhopadhyay and Linstedt presents manganese as a potential treatment for Shiga toxicosis by blocking Stx1-S trafficking. Proper trafficking through the cell is essential to Stx toxicity. After endocytosis, the Stx Ganoderic-acid-F holotoxin is trafficked from early endosomes to the Golgi apparatus and endoplasmic reticulum. In the ER, the enzymatic Asubunit separates from the holotoxin, is processed, and released into the cytosol where it inhibits protein synthesis by cleaving a conserved adenine in 28S ribosomal RNA. While the ER is the final destination of the holotoxin, trafficking through the Golgi is a required step. Mukhopadhyay and Linstedt conclude that HeLa cell protection against Stx1-S toxicity in the presence of manganese is due to altered trafficking; demonstrating that pretreating HeLa cells with 500 mM MnCl2 diverts trafficking of the Stx B-subunit from the Golgi to lysosomes, where it was subsequently degraded. When assayed using Stx1-S holotoxin, HeLa cells were protected in the presence of manganese. Moreover, the manganese treatment is reported to protect BALB/c mice from Stx1-S toxicity.
as Stx1-S and Stx2a. STEC can express one, or both forms of toxin. The reduced potency of Stx1 compared to Stx2a is well documented in mice and primates. Furthermore, Stx2a is more commonly associated with lifethreatening human disease; the majority of cases of HUS are associated with strains that produce Stx2a. Similarly, a recent report by Mukhopadhyay and Linstedt presents manganese as a potential treatment for Shiga toxicosis by blocking Stx1-S trafficking. Proper trafficking through the cell is essential to Stx toxicity. After endocytosis, the Stx Ganoderic-acid-F holotoxin is trafficked from early endosomes to the Golgi apparatus and endoplasmic reticulum. In the ER, the enzymatic Asubunit separates from the holotoxin, is processed, and released into the cytosol where it inhibits protein synthesis by cleaving a conserved adenine in 28S ribosomal RNA. While the ER is the final destination of the holotoxin, trafficking through the Golgi is a required step. Mukhopadhyay and Linstedt conclude that HeLa cell protection against Stx1-S toxicity in the presence of manganese is due to altered trafficking; demonstrating that pretreating HeLa cells with 500 mM MnCl2 diverts trafficking of the Stx B-subunit from the Golgi to lysosomes, where it was subsequently degraded. When assayed using Stx1-S holotoxin, HeLa cells were protected in the presence of manganese. Moreover, the manganese treatment is reported to protect BALB/c mice from Stx1-S toxicity.