HALT-C Folinic acid calcium salt pentahydrate investigators extended the study for up to an 8 year period of observation and found that the annual rate of initial liver-related complications was higher among the pegylated IFNa group than among the controls. Moreover, histologic features on sequential liver biopsies led the HALT-C investigators to speculate that pegylated IFNa might be associated with a long-term worsening of liver related morbidity in treatment nonresponders and in excess mortality among those with advanced liver disease. In one of the few large-scale studies to compare outcomes between IFNa treated and untreated patients, the Japanese IHIT Study Group followed patients who had been previously treated with IFNa monotherapy over a median period of 3.7 years, using paired biopsies to compare liver fibrosis progression among SVR patients, patients without SVR and untreated patients, stratified by fibrosis stage at initial biopsy. Among patients with initial METAVIR F2 or F3 fibrosis, a post hoc analysis of primary data presented in this report found no significant difference in cirrhosis development among patients without SVR and untreated patients. None of the patients with initial F2 or F3 fibrosis who achieved SVR developed cirrhosis. These data suggest that failed therapy may not increase the risk of cirrhosis during intermediate follow-up. Unlike the patients in the Japanese cohort, the majority of our patients were treated with IFNa/RBV combination therapy, rather than with IFNa monotherapy, and our follow-up period was more than twice as long. Our study included few Asian patients, who have a higher probability of achieving SVR, but also are more likely to progress to cirrhosis. Our finding that treatment failures have an increased long-term hazard of cirrhosis is thus neither directly supported nor contradicted by this or any other published report. Previously 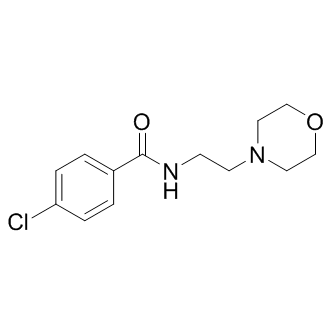 published studies also found that never treated HCV patients had a Chloroquine Phosphate greater mortality risk than patients who achieve SVR, and in some cases, those who fail treatment. In another IHIT study, Yoshida et al. found that the overall risk of death was reduced among IFNa treated patients, including treatment nonresponders, as compared to patients not receiving treatment. Their multivariate Cox proportional hazards models were adjusted by gender, age and IFNa therapy outcome. When survival analysis was further stratified by cirrhotic and noncirrhotic patients, IFNa therapy was associated with improved survival among the noncirrhotic patients only. A recent Cochrane Review of seven trials, including the HALT-C and EPIC3 studies, found a significant increase in all-cause mortality in IFNa maintenance patients and concluded that patients with severe fibrosis who failed previous IFNa treatment did not derive a survival benefit from further therapy with pegylated IFNa. These studies did not assess the effects of clinical and behavioral risk factors on liver disease outcomes as comprehensively as ours did. Our multivariate time-to-death analyses reveal that, even though nonresponders had more than twice the hazard of cirrhosis, their survival was not significantly different from that of never treated patients. The effects of cirrhosis on survival in our cohort may be offset by the relatively younger age and more beneficial clinical and psychosocial risk factor profile of nonresponders compared with never treated patients. Our findings suggest that some previously reported benefits of therapy among treatment failures might be attributable to the lower concomitant risks associated with treatment candidacy, rather than to disease modifying benefits of pharmacologic therapy.
published studies also found that never treated HCV patients had a Chloroquine Phosphate greater mortality risk than patients who achieve SVR, and in some cases, those who fail treatment. In another IHIT study, Yoshida et al. found that the overall risk of death was reduced among IFNa treated patients, including treatment nonresponders, as compared to patients not receiving treatment. Their multivariate Cox proportional hazards models were adjusted by gender, age and IFNa therapy outcome. When survival analysis was further stratified by cirrhotic and noncirrhotic patients, IFNa therapy was associated with improved survival among the noncirrhotic patients only. A recent Cochrane Review of seven trials, including the HALT-C and EPIC3 studies, found a significant increase in all-cause mortality in IFNa maintenance patients and concluded that patients with severe fibrosis who failed previous IFNa treatment did not derive a survival benefit from further therapy with pegylated IFNa. These studies did not assess the effects of clinical and behavioral risk factors on liver disease outcomes as comprehensively as ours did. Our multivariate time-to-death analyses reveal that, even though nonresponders had more than twice the hazard of cirrhosis, their survival was not significantly different from that of never treated patients. The effects of cirrhosis on survival in our cohort may be offset by the relatively younger age and more beneficial clinical and psychosocial risk factor profile of nonresponders compared with never treated patients. Our findings suggest that some previously reported benefits of therapy among treatment failures might be attributable to the lower concomitant risks associated with treatment candidacy, rather than to disease modifying benefits of pharmacologic therapy.