Studies on knock-out mice revealed that a loss of Pex11pa can be tolerated, with no obvious effect on peroxisome number or metabolism, whereas knock-out of Pex11pb causes neonatal lethality and is accompanied by several defects reminiscent of Zellweger syndrome. Very recently, the first patient with a defect in peroxisome division based on a homozygous non-sense mutation in the PEX11b gene was identified. In contrast to the severe clinical phenotype of the Pex11pb knock-out mice, the patient presented a milder phenotype with normal biochemical parameters of peroxisomes including, however, congenital cataracts, mild intellectual disability, progressive hearing loss, sensory nerve involvement, gastrointestinal problems and recurrent migrainelike episodes. To investigate the requirement of lipids 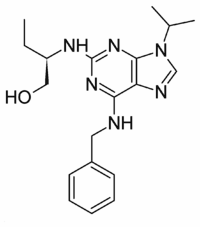 in Pex11pb-mediated membrane elongation and division, we cultured COS-7 cells stably expressing a GFP-fusion protein carrying a peroxisomal targeting signal under lipid- and serum-free conditions. At different time points, cells transfected with Myc-Pex11pb were analyzed by immunofluorescence microscopy using anti-Myc antibodies. Interestingly, cells cultured under lipid-free conditions revealed alterations in peroxisome morphology exhibiting enlarged, spherical organelles. These were reminiscent to the enlarged peroxisomes observed in fibroblasts from patients with defects in peroxisomal b-oxidation enzymes. In controls, the spherical peroxisomes are usually smaller, and elongated rodshaped or tubular peroxisomes are frequently observed in the cells. The latter is an indication for vivid growth and multiplication of the organelles, which appears to be reduced under lipid-free conditions. Remarkably, when Myc-Pex11pb was expressed, highly elongated membrane tubules were observed to extend from the large spherical peroxisomes, resembling ����balloons���� Gomisin-D connected to a string. This asymmetry was maintained over extended periods of time, which is unusual for Pex11pb-induced membrane elongation and division. Furthermore, the typical membrane constrictions were very rarely observed. Interestingly, Myc-Pex11pb was found to localize predominantly to the tubular membrane extensions and not to the globular peroxisomes, supporting its supposed function in membrane bending and deformation. These observations further indicate that Myc-Pex11pb can still generate and elongate membrane protrusions under lipid-free conditions. However, these do not result in proper division of the peroxisomal compartment. Thus, lipids likely contribute to the processes of membrane constriction and division. Taking into account the detergentsensitivity of Pex11pb, lipids may support the formation of Pex11pb complexes within the peroxisomal membrane, and may thus Folinic acid calcium salt pentahydrate modulate membrane elongation. Pex11 proteins in yeast, plant and animal cells contribute to the formation of peroxisomes and regulation of their abundance. Mammalian Pex11pb has been shown to elongate and proliferate peroxisomes in conjunction with the peroxisomal division machinery and has been proposed to possess membrane remodelling/deforming properties. Its loss is embryonically lethal in knockout mice. In humans, a first patient with a milder clinical phenotype but several disabilities has very recently been reported. Thus, there is currently great interest in the molecular and biochemical characterization of Pex11 proteins, their mode of action and regulation of peroxisome abundance.
in Pex11pb-mediated membrane elongation and division, we cultured COS-7 cells stably expressing a GFP-fusion protein carrying a peroxisomal targeting signal under lipid- and serum-free conditions. At different time points, cells transfected with Myc-Pex11pb were analyzed by immunofluorescence microscopy using anti-Myc antibodies. Interestingly, cells cultured under lipid-free conditions revealed alterations in peroxisome morphology exhibiting enlarged, spherical organelles. These were reminiscent to the enlarged peroxisomes observed in fibroblasts from patients with defects in peroxisomal b-oxidation enzymes. In controls, the spherical peroxisomes are usually smaller, and elongated rodshaped or tubular peroxisomes are frequently observed in the cells. The latter is an indication for vivid growth and multiplication of the organelles, which appears to be reduced under lipid-free conditions. Remarkably, when Myc-Pex11pb was expressed, highly elongated membrane tubules were observed to extend from the large spherical peroxisomes, resembling ����balloons���� Gomisin-D connected to a string. This asymmetry was maintained over extended periods of time, which is unusual for Pex11pb-induced membrane elongation and division. Furthermore, the typical membrane constrictions were very rarely observed. Interestingly, Myc-Pex11pb was found to localize predominantly to the tubular membrane extensions and not to the globular peroxisomes, supporting its supposed function in membrane bending and deformation. These observations further indicate that Myc-Pex11pb can still generate and elongate membrane protrusions under lipid-free conditions. However, these do not result in proper division of the peroxisomal compartment. Thus, lipids likely contribute to the processes of membrane constriction and division. Taking into account the detergentsensitivity of Pex11pb, lipids may support the formation of Pex11pb complexes within the peroxisomal membrane, and may thus Folinic acid calcium salt pentahydrate modulate membrane elongation. Pex11 proteins in yeast, plant and animal cells contribute to the formation of peroxisomes and regulation of their abundance. Mammalian Pex11pb has been shown to elongate and proliferate peroxisomes in conjunction with the peroxisomal division machinery and has been proposed to possess membrane remodelling/deforming properties. Its loss is embryonically lethal in knockout mice. In humans, a first patient with a milder clinical phenotype but several disabilities has very recently been reported. Thus, there is currently great interest in the molecular and biochemical characterization of Pex11 proteins, their mode of action and regulation of peroxisome abundance.