To gain further structural insight into these possibilities we developed two models of the caspase-6/ VEID-R110/3 ternary complex, one with unbound substrate to represent the Michaelis complex and one with substrate covalently bound to illustrate the tetrahedral intermediate. First, a model for the covalently bound tetrahedral intermediate was constructed by the covalent docking of a truncated substrate model to the caspase6/3 complex followed by attachment of the R110 fluorophore. This complex was then refined using Primeand MacroModel. The Michaelis complex model was derived by breaking the cysteine-substrate bond in the covalent model and performing a constrained optimization of the complex where the inhibitor, substrate and catalytic dyad residues were permitted to move freely. Both models provided low energy structures with plausible intermolecular contacts. Our existing data suggest that both mechanisms �C binding to the ternary complex and to the tetrahedral intermediate �C are important. With respect to MOI scenario #1, we observe cooperative binding of 3 with 2R110 or VEID-AMC to catalyticallydeadcaspase-6 by SPR. This result indicates that the 3/Michaelis complex can form, but it does not speak to whether 3 is able to prevent progress of the reaction, as would be required for inhibition. If 3 does indeed stabilize this complex to prevent formation of the tetrahedral intermediate, a possible mechanism is that 3 perturbs the oxyanion hole, inhibiting creation of the electrophilic carbonyl Regorafenib needed for attack. With respect to MOI scenario #2, our model also suggests that 3 could bind to the tetrahedral intermediate formed by addition of Cys163 to the amide bond. We observe by x-ray crystallography that the dimethoxy phenyl ring of 3 disrupts the water network around the catalytic His121. Thus it is possible that if 3 prevents collapse of the tetrahedral intermediate, it could do so by perturbing the local environment around this key residue, preventing it from acting as the general acid. Although we are unable to isolate and quantify the binding interactions of 3 to the tetrahedral intermediate, it is noteworthy that the measured affinities of 3 to the Michaelis complexand acyl enzymeare both weaker than the potency determined in enzymatic assays. We speculate that binding of 3 to the tetrahedral intermediate is the favored enzyme/substrate complex leading to potent inhibition. An unexpected feature of this inhibitor is the 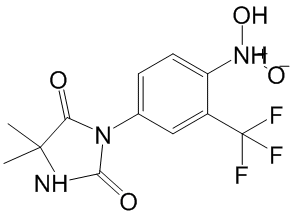 2�C3 orders of magnitude difference in inhibitory potency depending on the fluorophore employed in enzymatic assays, and the apparent lack of activity when fluorophore-free substrates are utilized. The computational models suggest one possible explanation for this difference, namely a polarized CH-p LY2109761 interaction between the paramethoxy group of 3 and the face of the orthogonal phenyl ring of the R110 dye, an interaction that is not possible with AMC-based substrates or substrates lacking a dye. The importance of such CH-p interactions has been noted previously. Furthermore, there appears to be either an edge-face or p-stack interaction between the phenyl ring of the inhibitor and the fluorophore aromatic ring. The remaining interaction energy difference can be explained by displacement of waters by the two extra rings of the R110, and/or additional hydrophobic interactions between the extra two rings of R110 and the protein. All of these interactions would be absent in a peptide substrate lacking a fluorophore at the P1′ position. It is known from studies on caspase-3 that prime side interactions can lead to a significant increase in inhibitory potency.
2�C3 orders of magnitude difference in inhibitory potency depending on the fluorophore employed in enzymatic assays, and the apparent lack of activity when fluorophore-free substrates are utilized. The computational models suggest one possible explanation for this difference, namely a polarized CH-p LY2109761 interaction between the paramethoxy group of 3 and the face of the orthogonal phenyl ring of the R110 dye, an interaction that is not possible with AMC-based substrates or substrates lacking a dye. The importance of such CH-p interactions has been noted previously. Furthermore, there appears to be either an edge-face or p-stack interaction between the phenyl ring of the inhibitor and the fluorophore aromatic ring. The remaining interaction energy difference can be explained by displacement of waters by the two extra rings of the R110, and/or additional hydrophobic interactions between the extra two rings of R110 and the protein. All of these interactions would be absent in a peptide substrate lacking a fluorophore at the P1′ position. It is known from studies on caspase-3 that prime side interactions can lead to a significant increase in inhibitory potency.