Tip60 also functions in the NF-kB pathway, via interactions with B-cell CLL/lymphoma 3 and cAMP-dependent signalling. Furthermore, Tip60 can function as a co-activator for a number of steroid hormone receptors including the AR, which is involved in the development and progression 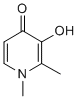 of prostate cancer. Studies have shown that AR can be acetylated by a number of HAT enzymes, including p300, p300/CBP-associated factorand Tip60, to increase its transcriptional activity. AR acetylation is thought to regulate the recruitment of co-activators to the transcriptional machinery of androgen responsive genes. In contrast, one report suggested that Tip60 is required to express the tumour metastasis MK-4827 suppressor KAI1 in CaP cell lines, suggesting that Tip60 is a tumour suppressor. Similarly, a Tip60 gene knockout study proposed Tip60 as a haplo-insufficient tumour suppressor at pre and early-tumoral stages of lymphoma, breast and head and neck cancers. However, studies on clinical prostate specimens contradict this suggestion and support Tip60 as an oncogene in CaP. Thus, targeting the VE-822 acetylase activity of Tip60 could be a useful therapeutic strategy in CaP. A small number of HAT inhibitors have been reported. Coupling a histone H3 peptide to CoA to form a bisubstrate inhibitor of HAT activity has been described; however, the compound has poor cell membrane permeability. The natural products anacardic acid and garcinol are HAT inhibitors that are cell permeable; they sensitise cells to IR, which could be useful as a combination therapy for cancer treatment. Other inhibitors of HAT function include a-methylene butyrolactones, benzylidene acetonesand alkylidene malonates. More recently, isothiazolones, which covalently bind to the HAT active site thiol, have been described as an effective starting point for molecular modelling-based approaches for generating more potent and specific inhibitors. In the current study we employed a high throughput screening approach to identify selective inhibitors of Tip60. Based on the lead molecule, structurally related compounds were generated and tested for HAT inhibition and Tip60 specificity in order to identify a molecular tool for studies in cell line models of CaP. Protein acetylation, as a regulatory mechanism, is proving to be important in many cellular pathways, not just gene transcription via histone modification. Both sets of enzymes responsible for regulating acetylation, HATs and HDACs, are de-regulated in disease states. Therefore, targeting both types of enzymes with small molecule inhibitors as a therapeutic strategy is valid. Inhibitors against HDACs have been found to be successful in clinical trials; however, HAT inhibitors are at an earlier stage of development. Recently, there have been some putative HAT inhibitors described, although none appear able to distinguish significantly between the different HAT family members and none have been specifically developed against Tip60, a HAT enzyme which appears to play a particular role in CaP development and progression. To address this point, we identified a HAT inhibitor, using HTS and targeted compound synthesis, which inhibits Tip60 over other HAT enzymes. The requirement to fully validate HTS hits through resynthesis is widely accepted as material in commercial compound collections may include unidentified impurities, or may degrade on storage, typically as frozen DMSO solutions, giving false positives. In this case, a literature synthesis for 1 was not available and a route had to be developed. The first scheme attempteddid not give the target compounds, 1, or its desmethyl analogue; however, the isocyanato and disulfide analogues 4�C7 were prepared.
of prostate cancer. Studies have shown that AR can be acetylated by a number of HAT enzymes, including p300, p300/CBP-associated factorand Tip60, to increase its transcriptional activity. AR acetylation is thought to regulate the recruitment of co-activators to the transcriptional machinery of androgen responsive genes. In contrast, one report suggested that Tip60 is required to express the tumour metastasis MK-4827 suppressor KAI1 in CaP cell lines, suggesting that Tip60 is a tumour suppressor. Similarly, a Tip60 gene knockout study proposed Tip60 as a haplo-insufficient tumour suppressor at pre and early-tumoral stages of lymphoma, breast and head and neck cancers. However, studies on clinical prostate specimens contradict this suggestion and support Tip60 as an oncogene in CaP. Thus, targeting the VE-822 acetylase activity of Tip60 could be a useful therapeutic strategy in CaP. A small number of HAT inhibitors have been reported. Coupling a histone H3 peptide to CoA to form a bisubstrate inhibitor of HAT activity has been described; however, the compound has poor cell membrane permeability. The natural products anacardic acid and garcinol are HAT inhibitors that are cell permeable; they sensitise cells to IR, which could be useful as a combination therapy for cancer treatment. Other inhibitors of HAT function include a-methylene butyrolactones, benzylidene acetonesand alkylidene malonates. More recently, isothiazolones, which covalently bind to the HAT active site thiol, have been described as an effective starting point for molecular modelling-based approaches for generating more potent and specific inhibitors. In the current study we employed a high throughput screening approach to identify selective inhibitors of Tip60. Based on the lead molecule, structurally related compounds were generated and tested for HAT inhibition and Tip60 specificity in order to identify a molecular tool for studies in cell line models of CaP. Protein acetylation, as a regulatory mechanism, is proving to be important in many cellular pathways, not just gene transcription via histone modification. Both sets of enzymes responsible for regulating acetylation, HATs and HDACs, are de-regulated in disease states. Therefore, targeting both types of enzymes with small molecule inhibitors as a therapeutic strategy is valid. Inhibitors against HDACs have been found to be successful in clinical trials; however, HAT inhibitors are at an earlier stage of development. Recently, there have been some putative HAT inhibitors described, although none appear able to distinguish significantly between the different HAT family members and none have been specifically developed against Tip60, a HAT enzyme which appears to play a particular role in CaP development and progression. To address this point, we identified a HAT inhibitor, using HTS and targeted compound synthesis, which inhibits Tip60 over other HAT enzymes. The requirement to fully validate HTS hits through resynthesis is widely accepted as material in commercial compound collections may include unidentified impurities, or may degrade on storage, typically as frozen DMSO solutions, giving false positives. In this case, a literature synthesis for 1 was not available and a route had to be developed. The first scheme attempteddid not give the target compounds, 1, or its desmethyl analogue; however, the isocyanato and disulfide analogues 4�C7 were prepared.