Formulations were removed from consideration if they were defined by the panel as having ��very low�� to ��low�� in mano tensile strength, as these formulations could not be handled, or if their pliability was defined as ��low�� to ��moderate��, as these formulations would not allow for any flexibility when applied. Therefore, formulation series D, specifically subformulation ��D3�� was identified as the lead film formulation for Dinaciclib 779353-01-4 development in this study. The resulting film was a smooth translucent film that can easily conform to the contours of the arm with a thickness of 150 ��m and a drug loading of 448 �� 22.1 ��g/cm2. The transdermal films were manufactured to have a water content of 1-5% to produce a stable polymer film matrix but still allow for enough pliability to avoid issues with the films being dry and brittle. The film formulation under development had a water content of 1.51 �� 0.26% which corresponds to 0.19 ��L/cm2 of water. Overall, the films showed significant swelling when exposed to high levels of humidity. At a 95% relative humidity environment, the films resulted in swelling of 430% from a completely dried film. However, under ambient MDV3100 conditions, the films only resulted in a swelling of 8.35%. The primary excipient in the films is ethyl cellulose, a hydrophobic polymer, which will limit film hygroscopy and swelling. However, the inclusion of HPMC, a hydrophilic polymer, is responsible for resulting in a film that is water-permeable and subject to swelling. This hydration loosens the polymer matrix which then allows for the drug to be released from the film. When sealed into packaging, the film resulted in no increase of water content when stored at 30��C / 65% R.H. and 40��C / 75% R.H. for up to 3 months. In the dissolution media, the cumulative amount of IQP-0410 recovered from the film formulation was near 100%. In films immediately tested and films tested over 3 months that were stored at standard and accelerated conditions, all films resulted in complete IQP-0410 release and recovery after 26 hours. The rapid release of IQP-0410 from the films in the dissolution media could be explained by the hydrophobic nature of the ethyl cellulose. While ethyl cellulose limits film hygroscopy, it readily solubilizes in non-aqueous solutions such as ethanol. Therefore, with a dissolution media containing both ethanol and water, the entire film is rapidly swelling to allow for a rapid release of IQP-0410. Another reason for the rapid in vitro release is the inclusion of Di-n-butyl phthalate, which has been demonstrated to enhance in vitro release. There was observed a minor increase in the release rate of IQP-0410 from the films stored under accelerated conditions. While not significant, it is was observed that these films in mano were more pliable that the films stored at standard conditions. The increased pliability due to the heat may reduce the integrity of the film polymer matrix and may contribute to the slightly faster release of IQP-0410 into dissolution media measured; however, the cumulative recovered IQP-0410 was unaffected. This rapid release rate, however, shouldn��t be indicative of the actual release of IQP-0410 from the transdermal film when applied to a barrier as optimally there will be little media when the films are applied to cause premature drug release. Regardless, these in vitro release studies demonstrate that formulation of IQP-0410 into the polymeric transdermal 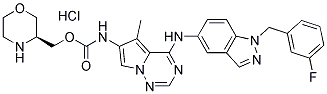 films does not negatively affect API recovery. Additionally, the films manufactured showed a uniform distribution of IQP-0410 through the film with an RSD of < 5.29% overall. The in vitro / ex vivo release and permeability studies of IQP-0410 from the transdermal films were performed on synthetic PVDF membranes and epidermal tissues, respectively. When applied to the membrane and moistened, the transdermal films displayed a linear release of IQP-0410 across the membrane into 1:1 IPA/PBS solution. While the flux of IQP-0410 across the membrane is not a true measurement of drug delivery and permeability, the drug transport of IQP-0410 from the transdermal film across the membrane does correspond to a zero-order release kinetic profile. Therefore, with a calculated flux of 9.83 ��g/cm2/hr, we calculate a potential complete release of IQP-0410 through the membrane in 1.75 days. When applied to epidermal tissue for 3 days, the transdermal films resulted in a linear zero-order release rate through the tissue into the basal media.
films does not negatively affect API recovery. Additionally, the films manufactured showed a uniform distribution of IQP-0410 through the film with an RSD of < 5.29% overall. The in vitro / ex vivo release and permeability studies of IQP-0410 from the transdermal films were performed on synthetic PVDF membranes and epidermal tissues, respectively. When applied to the membrane and moistened, the transdermal films displayed a linear release of IQP-0410 across the membrane into 1:1 IPA/PBS solution. While the flux of IQP-0410 across the membrane is not a true measurement of drug delivery and permeability, the drug transport of IQP-0410 from the transdermal film across the membrane does correspond to a zero-order release kinetic profile. Therefore, with a calculated flux of 9.83 ��g/cm2/hr, we calculate a potential complete release of IQP-0410 through the membrane in 1.75 days. When applied to epidermal tissue for 3 days, the transdermal films resulted in a linear zero-order release rate through the tissue into the basal media.