STAT5A1*6 has two amino acid substitutions and it is constitutively phosphorylated, localized in the cell nucleus and transcriptionally active in the absence of IL-3. In the BaFiso system presented here, the protective potential of myr-Akt is slightly greater than that provided by STAT5A1*6, which may be explained by the greater expression of myr-Akt. The design of the screen relies on the lack of relevant crosstalk between the pathways engineered to support IL-3 independent survival. Previous work has shown that the induced expression of bcl-xL and pim-1 promotes the IL-3-independent survival of Ba/F3 cells upon activation of STAT5. In contrast, studies in multiple cell lines suggest that Akt phosphorylates and inactivates proapoptotic proteins such as GSK-3b, Foxo3a and Bad in response to IL-3. We confirmed that the activation of Stat5 signaling in BCS cells did not increase Akt activity either in the presence or absence of IL-3. Another common source of interference to be SP600125 JNK inhibitor mitigated in multiplexed screening procedures is the bleed-through of fluorescence from one BIBW2992 EGFR/HER2 inhibitor channel to the other. BaFiso allows simultaneous viewing of three different fluorescent signals and sharp separation of the emission signals from the cyan and yellow protein is achieved using a special filter set. We implemented BaFiso as an automated live-cell assay using a multidrop dispenser, a robotic workstation and a robotic cell imaging platform. We assessed the properties of this HTS co-culture assay using a panel of test compounds of known activity. The cytotoxicity of the test compounds was monitored by quantifying the DRAQ5 labelled cells and all compounds tested except LiCl and Minerval reduced the viability 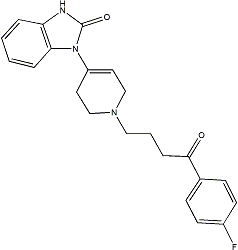 of Ba/F3 cells. The fact that only two compounds known to selectively interfere with Akt signaling, Akt inhibitor X and UCN-01, reduced the number of yellow tagged BYA cells demonstrates the specificity of the BaFiso system. The Akt inhibitor X is a N-substituted phenoxazine that inhibits the activity of Akt even in the absence of its pleckstrin homology domain and it has been suggested that it may bind in the ATP binding site. In contrast, UCN-01 has been reported to inhibit several kinases including PDK1, a key regulator of Akt activity. Interestingly, staurosporine that differs from UCN-01 only by the absence of a hydroxy group on the lactam ring failed to change the ratio of the BaFiso cell lines. A specificity analysis against a kinase panel revealed different patterns of inhibition for UCN-01 with respect to staurosporine. It remains to be determined if these differences in specificity could account for the different behaviour observed for these two compounds in the BaFiso assay. The BaFiso screening design presented here offers some major advantages over traditional in vitro biochemical assays or more classical cellular assays. Co-culture and simultaneous testing of the paired isogenic cell lines in this assay provides an internal control and eliminates errors resulting from separate assessments. BaFiso is an image based high throughput assay that enables compound that produce artefacts and cytotoxicity to be identified on a single cell basis. Live cell imaging of the BaFiso cell lines permits the repeated monitoring of the same cells over the timecourse of an experiment, leading to a more accurate assessment that minimizes the variability in cell numbers between wells. Finally, the dual fluorescence co-culture system used in BaFiso is adaptable to any gene or pathway that can support IL-3 independent survival of Ba/F3 cells. Friedreich ataxia is an inherited recessive disorder characterized by progressive neurological disability and heart disease. Onset is usually in childhood, but it may vary from infancy to adulthood. Atrophy of sensory and cerebellar pathways causes ataxia, dysarthria, fixation instability, deep sensory loss and loss of tendon reflexes.
of Ba/F3 cells. The fact that only two compounds known to selectively interfere with Akt signaling, Akt inhibitor X and UCN-01, reduced the number of yellow tagged BYA cells demonstrates the specificity of the BaFiso system. The Akt inhibitor X is a N-substituted phenoxazine that inhibits the activity of Akt even in the absence of its pleckstrin homology domain and it has been suggested that it may bind in the ATP binding site. In contrast, UCN-01 has been reported to inhibit several kinases including PDK1, a key regulator of Akt activity. Interestingly, staurosporine that differs from UCN-01 only by the absence of a hydroxy group on the lactam ring failed to change the ratio of the BaFiso cell lines. A specificity analysis against a kinase panel revealed different patterns of inhibition for UCN-01 with respect to staurosporine. It remains to be determined if these differences in specificity could account for the different behaviour observed for these two compounds in the BaFiso assay. The BaFiso screening design presented here offers some major advantages over traditional in vitro biochemical assays or more classical cellular assays. Co-culture and simultaneous testing of the paired isogenic cell lines in this assay provides an internal control and eliminates errors resulting from separate assessments. BaFiso is an image based high throughput assay that enables compound that produce artefacts and cytotoxicity to be identified on a single cell basis. Live cell imaging of the BaFiso cell lines permits the repeated monitoring of the same cells over the timecourse of an experiment, leading to a more accurate assessment that minimizes the variability in cell numbers between wells. Finally, the dual fluorescence co-culture system used in BaFiso is adaptable to any gene or pathway that can support IL-3 independent survival of Ba/F3 cells. Friedreich ataxia is an inherited recessive disorder characterized by progressive neurological disability and heart disease. Onset is usually in childhood, but it may vary from infancy to adulthood. Atrophy of sensory and cerebellar pathways causes ataxia, dysarthria, fixation instability, deep sensory loss and loss of tendon reflexes.